Chemical Energetics Formulas
If you are struggling to solve problems on Chemical Energetics you can take help from the Simple Chemical Energetics Formulas listed. Make your work simple by accessing the Cheat Sheet for Chemical Energetics and use them while solving your problems. The below listed Chemical Energetics Formulae will familiarize you with the concept in a better way.
Solve your chemistry problems fastly and efficiently taking the help of Chemistry Formulas and learn about the Concepts without much effort.
Formulas List of Chemical Energetics
Revise the Concepts of Chemical Energetics completely & thoroughly to solve the related problems at a faster pace. You can develop deeper knowledge on the fundamentals of Chemical Energetics after going through the formulae list compiled over here. Solve numerous problems so that you can recall the Chemical Energetics Formulas simply rather than by hearting.
1. System:
- Open: [exchange matter and energy] with the surroundings.
- Closed: [exchanged only energy] with the surroundings.
- Isolated: [exchanged neither matter nor energy] with the surroundings.
2. E, Internal energy and ΔE:
E = Et + Ev + Er + Ee + En + Ep etc.
ΔE = Ep – ER [R → P]
3. H1 Enthalpy or heat content and ΔH:
ΔH = Hp – HR [R → P]
ΔH = – ve (exothermic)
ΔH = +ve (endothermic)
4. Work and Heat (Sing convention):
☞ work done by the system is negative
☞ work done on the system is positive
☞ heat observed by the system is positive
☞ heat observed by the surroundings is negative
5. First Law:
ΔE = Q + W (SI)
ΔE = Q – W (non-SI)
6. Expression for pressure volume work:
W = -PΔV
7. Maximum work in a reversible expansion:
W = -2.303 n RT log \(\frac{V_{2}}{V_{1}}\)
= – 2.303 nRT log \(\frac{P_{1}}{P_{2}}\)
Wrev ≥ Wirr
8. Isothermal process:
ΔE = 0, ΔH = 0, Q = -W
9. Isochoric process:
W = 0, Q = ΔE
10. Adiabatic process:
Q = 0, ΔE = W
11. Cyclic process:
ΔE = 0
q = – W
12. Isothermal isobaric process:
= Q – W,
ΔE = 0, ΔH = 0
13. Adiabatic reversible expansion:
Tvγ-1 = constant
PVγ = constant [γ = \(\frac{C_{p}}{C_{v}}\)]
14. Enthalpy and heat content:
ΔH = ΔE + PΔV [q(p) = q (v) + ΔngRT]
ΔH = ΔE + ΔngRT [Δng = np – nr]
15. Unit for heat (cal. and joule):
1 cal = 4.184 J
1 Lt-atm = 101.3 J = 24.206 cal
16. KirchofPs equation:
ΔET2 = ΔET1 + ΔCV (T2 – T1) [constant V]
ΔHT2 = ΔHT1 + ΔCP (T2 – T1) [constant P]
17. Entropy(s):
Measure of disorder or randomness
ΔS = ΣSp – ΣSR
ΔS = \(\frac{\mathrm{q}_{\mathrm{rev}}}{\mathrm{T}}\) = 2.303 nR log \(\frac{V_{2}}{V_{1}}\)
= 2.303 nR log \(\frac{P_{1}}{P_{2}}\)
18. Fusion process:
ΔS = \(\frac{\Delta \mathrm{H}_{\text {fus. }}}{\mathrm{T}_{\mathrm{f}}}\)
☞ Vapourisation process
ΔS = \(\frac{\Delta \mathrm{H}_{\mathrm{vap}}}{\mathrm{T}_{\mathrm{b}}}\)
☞ For reversible process:
ΔSsys + ΔSsurr = 0
☞ For an irreversible process: ΔSsys + ΔSsurr > 0
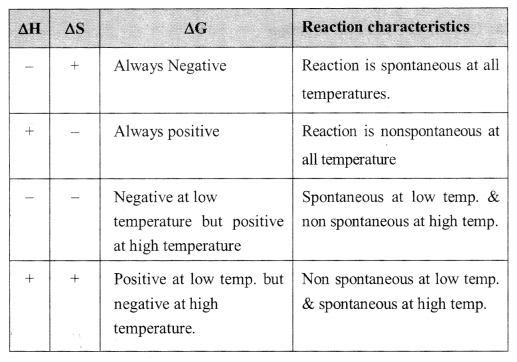
19. Free energy change:
ΔG = ΔH – TΔS
ΔG < 0 (spontaneous) [-ve] ΔG = 0 (equilibrium) ΔG > 0 (non-spontaneous) [+ve]
– ΔG is measure at useful work
– ΔG = W (maximum) – PΔV
☞ ΔG° = – 2.303 RT log K [K = equilibrium constant]
20. Clapeyron – Clausius equation|
log\(\frac{P_{2}}{P_{1}}=\frac{\Delta H_{v}}{2.303 R}\left(\frac{T_{2}-T_{1}}{T_{2} T_{1}}\right)\)
ΔHV = Latent heat of vapourisation
21. Third law Lt T → 0, S = 0 [for a perfect crystalline substance]
☞ Carnot’s cycle (second law): Efficiency of heat engine:
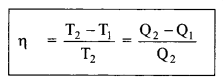
22. Different forms of heat of reaction:
Heat of combustion, heat of hydration, heat of neutralization, heat of fusion, heat of vapourisation, heat of sublimation etc.
(Enthalpy of reaction: R → P
ΔH° = ΣΔH°r(P) – ΣΔH°f(Rf)
23. Hess’s law: (Path-1)
ΔH = ΔH1 + ΔH2 (Path-II)
24. Heat of formation:
Change in heat content when one mole of the substance is formed from its element.
ΔH°298 = H°compound
[standard enthalpy of free element is taken as zero]
25. Hear of reaction from bond energy:
ΔH = [sum of BE of reactants] – [sum of BE of products]
☞ Bom Haber cycle is used to determine lattice energy.
☞ Heat capacity
Cp = \(\left(\frac{\partial \mathrm{H}}{\partial \mathrm{T}}\right)_{\mathrm{p}}\), Cv = \(\left(\frac{\partial \mathrm{E}}{\partial \mathrm{T}}\right)_{\mathrm{v}}\)
Cp – Cv = R [Cp > Cv]
γ = \(\frac{C_{p}}{C_{v}}\)
☞
Avail one-stop destination for formulae of different subjects from Onlinecalculator.guru and be thorough with the concepts.