Hydrogen And It's Compounds Formulas
Do you have a grip on the concept of Hydrogen and It's Compounds? Then, this page gonna your one-stop destination to learn all the concepts easily. Here we have curated a list of Hydrogen And It's Compounds Formulas to understand the equations or reactions related to hydrogen and make the calculations so simple & quick. Want to check the Hydrogen And It's Compounds Formulae Sheet or Tables then go ahead and take a look.
Solve your chemistry problems fastly and efficiently taking the help of Chemistry Formulas and learn about the Concepts without much effort.
List of Hydrogen And It's Compounds Formulas | Hydrogen And Its Compounds Formula Sheet
In Hydrogen And It's Compounds concept, the most important formulas to be aware of are the Preparation of hydrogen, Properties of molecular hydrogen, Isotopes of hydrogen, and many more. Learn and remember all Hydrogen And It's Compounds Formulas by using the formulae sheet and tables provided here below.
1. Preparation of hydrogen:
(i) Passing steam over hot iron (Lane process)
3Fe + 4H2O → Fe3O4 + H2 ↑
(ii) By the action of water on hydrolith
CaH2 + H2O → Ca(OH)2 + 3H2
(iii) By the electrolysis of water
(iv) Bosch process
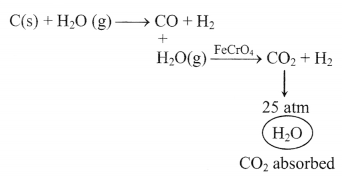
2. Properties of molecular hydrogen:
Metals like Pd, Pt, Ni, Co etc. adsorb large quantities of the gas due to vacancies between these atoms. This is known as “occlusion”.
3. Reaction with vegetable oils:
![]()
This process is known as “hydrogenation” or “hardening of oils”
3. Isotopes of hydrogen:

4. Different forms of hydrogen:
Atomic hydrogen:

Nascent hydrogen:
Zn + dil.H2SO4 → ZnSO4 + 2H
Ortho and Para hydrogen:
- If two nuclei have same spin then it is called “Ortho H2“.
- If two nuclei have different spin then it is called “Para H2“.
5. HYDRIDES:
(i) Ionic or salt like hydrides: s block
Li H, NaH, KH. RbH, SrH2, BaH2 etc.
Be & Mg hydrides are covalent in nature
(ii) Molecular or covalent hydrides: p blcok
NH3, PH3, H2O, CH4 etc.
(iii) Metallic or Interstitial hydrides:
trasition elements
In these hydrides, hydrogen atoms occupies the interstitial position of
metallic lattice.
La H2.87, Ti H1.8, Zr H1.9
Hydride gap = 7, 8, 9
6. Water:
(i) The existence of hydrogen bonding is responsible for high values of specific heat, the latent of fusion and latent heat of vapourisation and high boiling point.
(ii) Some of the covalent compounds such as alcohols, carboxylic acids and carbohydrates are soluble in water due to formation of hydrogen bonding.
7. Hardness of Water:
• temporary hardness of water is due to bicarbonates of Ca and Mg. It can be removed by –
- Boiling
- Clark method using CaO
• Permanent hardness of water is due to chloride/sulphate of Ca and Mg. It can be removed by –
- Adding washing soda, Na2CO3
- Calgon [Na6(PO3)6]
- Permutit, Na2 Al2 Si2 O8. xH2O
- Artificial resins, RSO3H & RNH2OH
8. Heavy water or Deuterium oxide (D2O):
- It is produced by repeated electrolysis of ordinary water containing alkali.
- Most important use of heavy water is in nuclear reactors for slow down the speed of neutrons (i.e. as a moderator)
9. Hydrogen Peroxide
Preparation:
- BaO2 . H2O + H2SO4 → BaSO4 + H2O2 + 8 H2O
- Na2O2 + 2 H2SO4 → 2 Na2SO4 + H2O2
- BaO2 + H2O + CO2 → BaCO3 + H2O2
- Oxidation of 2Alkyl anthraquinol
- Electrolysis of 50% H2SO4
10. Chemical properties:
(a) Decomposition:
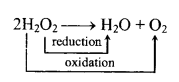
(b) H2O2 can accept as well can donate electrons and thus it can acts as an oxidising and reducing agent in acidic as well as alkaline medium
(c) Oxidising properties: It is a strong oxidising agent under acidic and alkaline conditions. Oxidation in acidic medium is generally slow while rapid in alkaline solution.
(i) In acidic solution:
H2O2 + 2H+ + 2e → 2 H2O
(ii) In alkaline solution:
H2O2 + 2OH–+ 2e → 2 H2O + 2O2-
(d) Reducing properties:
It acts as a reducing agent towards strong oxidising agents in acidic as well as alkaline medium. Reducing action in acidic solution is slower than in alkaline solution.
(e) Bleaching action:
H2O2 → H2O + [O]
Structure:
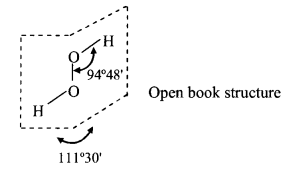
Structure of H2O2 in the gas phase.
11. Tests of H2O2:
(a) It liberates iodine from KI solution in the presence of ferrous sulphate.
(b) It gives orange red colour with acidified titanium oxide due to formation of pertitanic acid.
Ti(SO4)2 + H2O2 + 2H2O → H2TiO4 + 2H2SO4
(c) It gives blue colour with acidified K2Cr2O7 and ether. The blue colour of chromium peroxide is stabilized by ether.
CrO42- + 2H+ + 2H2O2 → CrO5 + 3H2O
Make the most out of the Hydrogen And It's Compounds Formulae provided at Onlinecalculator.guru and get your calculations or reactions simple & fast.