Metals & Metallurgy Formulas
Have you memorized all Metals & Metallurgy Formulas very well? If not, then this would be the right time and right page for you. We as a team provided the collective Metals & Metallurgy Formulas list on this page for a better understanding of the concept to all students. By referring to the formulae sheet of Metals & Metallurgy, you can easily grasp the concept and make your questions all answered at a faster pace.
Solve your chemistry problems fastly and efficiently taking the help of Chemistry Formulas and learn about the Concepts without much effort.
Metals & Metallurgy Formulae List | Important Ore & Formula Table for Metals & Metallurgy
The important and standard formulas of the Metals & Metallurgy concept are listed here by keeping the student's level of understanding & knowledge. Right from the introduction to properties of metals concepts formulas are explained & given here in the form of list & tables. You can make the most out of this Metals & Metallurgy Formula Tables and improve your subject knowledge and make your calculations done easily & quickly.
INTRODUCTION:
The process of extraction of metal from its ores in profitable manner.
- Mineral is a substance in which metal is present in either native state or combined state.
- ‘‘Ore” is the mineral from which the metal can be economically and conveniently extracted.
- “Gangue or matrix” is the non metallic impurities present in the ore.
IMPORTANT ORES
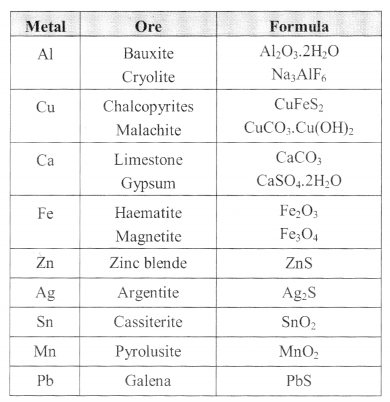
Process of metallurgy involves –
- Concentration
- Isolation
- Purification
1. Concentration:
- Magnetic separation for ores of iron.
- Froath floatation for sulphide ores.
- Leaching (hydromatallurgy): Bayer’s process for A1 & cyanide process for Au and Ag.
2. Isolation:
(i) Calcination:
(Conversion of the concentrated ore into its oxide form):
- Ore is heated in absence of air to remove water or CO2 from hydrated oxides or carbonates respectively
- Process temperature is below the melting points of treated ores
- During calcination moisture, volatile impurities are removed there by ore becomes porous
Ex. 2Fe2O3.3H2O → 2Fe2O3 + 3H2O
CaCO3 → CaO + CO2
(ii) Roasting (Conversion of the concentrated ore into its oxide form): Employed for sulphide ores
- Ore is heated strongly with other substances, usually with oxygen
- Process temperature is below the melting points of treated ore
- Some of the impurities removed as volatile substances
S + O2 → SO2↑
a. Reduction by carbon (smelting)
The oxides of less electropositive metals like Pb, Zn, Fe, Sn, Cu etc. are reduced by strongly heating with coal or coke. Reduction of the oxide with carbon at high temperature is known as smelting.
b. Reduction by aluminium (Alumino-Thermic reduction)
- Aluminium acts as reducing agent due to its high electropositive nature.
- Oxides such as Cr2O3, Mn3O4 are reduced by this method because carbon or CO are not efficiently reduced.
- The process is also known as “Gold Schmidt thermite process”.
c. Reduction by heating in air (Auto-reduction)
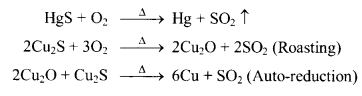
d. Electrolytic reduction (Electro-metallurgy)
(i) Employed for highly electropositive metals such as Na, K, Ca, Mg, A1 etc.
(ii) These metals are extracted by the electrolysis of their oxides, hydroxides or chlorides in fused state
on fusion: NaCl ⇌ Na+ + Cl– (ions become mobile)
on electrolysis:
at cathode: Na+ + e– → Na
at anode: Cl– → Cl + e–
Cl + Cl → Cl2
e. Hydrometallurgy (Reduction by precipitation)
Process in which more electropositive metals displace less electropositive metals from salt solution. Silver sulphide dissolved in sodium cyanide which forms a soluble complex, then silver is precipitated by the addition of zinc powder.
Ag2S + 4 NaCN → 2 Na [Ag (CN)2] + Na2S(sodium dicyanoargentate (I))
2 Na [Ag (CN)2] + Zn → Na2 [Zn (CN)4] + 2 Ag ↓
3. Refining or purification:
- The metals after reduction process consists of number of impurities like Si, P, slag, oxides, other metals etc.
- Removal of all these impurities to get pure metal is called as refining.
1. Liquation:
- This is based on the principle of difference in melting points of metal and impurity
- Employed for purification of low melting point metals like Pb, Sn etc.
2. Distillation process:
- This is based on difference in boiling points of metals and impurities.
- Employed for low boiling point metals like Zn, Hg etc.
3. Oxidation process
- The impurities converted into oxide & skimmed off from the metal.
- Various oxidation processes used for different metals bear different names, e.g., poling, pudding, bessemerisation and cupellation (for Ag).
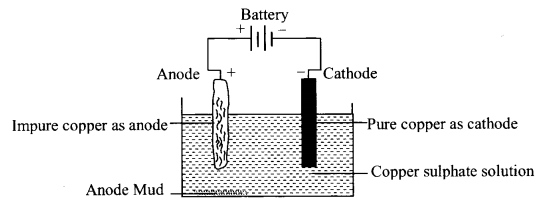
4. Electrorefining:
Employed for refining of highly electro positive metals like Al, Cu, Ag, Zn, Sn, Pb, Cr and Ni.
Electrolytic refining of copper:
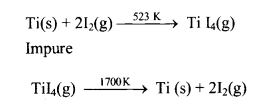
5. Van-Arkel process:
- In this method, the metal is converted into a volatile unstable compound (e.g. iodide), and impurities are not affected during compound formation.
- Employed for purification of metals like titanium and zirconium.
6. Zone refining:
- Employed for metals which requires in very high purity like semi conductors, such as Si, Ge
- The method is based on the principle that an impure metal on solidification will deposit crystals of pure metal and the impurities will remain behind in the molten part of the metal
7. Mond’s process:
Nickel is purified by using CO gas. This involves the formation of nickel tetracarbonyl.
IRON:
(a) Ores:
Haematite – Fe2O3 (chief); Limonite – 2Fe2O3.3H2O ; Siderite – FeCO3;
Magnetite – Fe3O4, Pyrite – FeS2.
(b) Process:
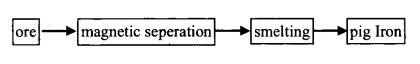
(c) Reactions:
(i) Roasting: FeO changes to Fe2O3 to prevent the loss of iron during smelting.
4FeO + O2 → 2Fe2O3
(ii) Smelting: In reduction zone:
At 400° C
3Fe2O3 + CO → 2Fe3O4 + CO2↑
Fe3O4 + CO → 3FeO + CO2 ↑
At 600°C
FeO + CO → Fe + CO2↑
Ca CO3 → CaO + CO2↑
In central zone:
Fe + 2CO → CO2 + C + Fe
(900 – 1200°C) Fe acts as catalyst here and ‘C’ so formed is dissolved in Fe.
CaO (flux) + SiO2(impurity) → CaSiO3(slag)
In fusion zone:
CO2 + C → 2CO, ΔH = + ve
(1100- 1200°C) melting of iron takes place
In combustion zone:
C + O2 → 2CO, ΔH = – ve
(1500- 1600°C)
(d) Pig Iron: C 3.1 – 4.5%, small amounts of Si, S, P ; hard and brittle, obtained from blast furnace
(e) Wrought Iron: C 0.15 – 0.28%, purest form ; malleable, fibrous
(f) Steel: C 0.15 – 1.5%, strength is high.
COPPER:
(a) Ores:
Copper pyrites CuFeS2 (chief) ; cuprite or ruby copper Cu2O ; copper glance Cu2S; malachite Cu (OH)2 . CuCO3 ; Azurite Cu (OH)2 . 2CuCO3.
(b) Process:
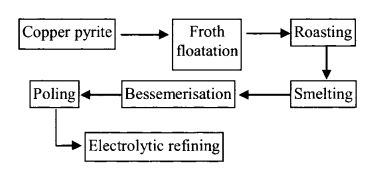
(c) Reactions:
(i) Roasting:
2CuFeS2 + O2 → Cu2S + 2FeS + SO2
2FeS + 3O2 → 2FeO + 2SO2
2Cu2S + 3O2 → 2Cu2O + 2SO2
(ii) Smelling:
Cu2O + FeS → Cu2S + FeO
FeO + SiO2 → FeSiO3
(iii) Bessemerisation :
2FeS + 3O2 → 2FeO + 2SO2
FeO + SiO2 → FeSiO3
2Cu2S + 3O2 → 2CuO + 2SO2
Cu2S + 2Cu2O → 6Cu2+ + SO2 (self reduction)
(iv) Poling: Molten Cu is stirred with poles of green wood to reduce any copper oxide in Cu.
(v) Electrolytic refining: Anode-impure Cu; cathode – pure Cu; electrolyte CuSO4 + H2SO4.
ALUMINIUM:
(a) Ores:
- Oxides : Bauxite Al2O3.2H2O(chief) ; Diaspore Al2O3.H2O ; corundum Al2O3.
- Fluorides : Cryolite Na3AlF6.
(b) Process:
(i) Purification of Bauxite:
Baeyer’s Method:
If Fe2O3 is major impuirity – Red bauxite
– ore is roasted to convert ferrous oxide to ferric oxide
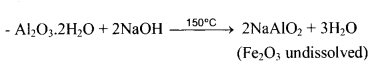
– NaAlO2 + 2H2O → NaOH + Al(OH)3
white ppt.
![]()
(ii) Electrolysis of fused Alumina.
Cathode : Iron-tank lined with carbon bricks carbon rods
Anode : carbon rods
Electrolyte : Molten [Al2O3 (5%) Na3AlF6 (85%) + CaF2(5%) + AlF3(5%)] O2 is liberated at anode and A1 collects at the bottom.
(iii) Reactions : Na3AlF6 → 3NaF + AlF3
AlF3 ⇌ Al3+ + 3F–
At anode : Al2O3 + 6F– → 2Al F3 + 3/2 O2 + 6e–
At cathode : 2Al3+ + 6e– → 2Al
(iv) Electrolytic refining (Floope’s process), three layers process.
Cathode : Carbon electrodes
Anode : Fe tank lined with
Bottom layer : Impure aluminium consists of Cu, Si etc in molten state.
Middle layer : molten mixture of Fluorides of Na, Ba, Al and Al2O3
Top layer : pure molten aluminium.
On passing the current, Al is deposited at cathode from the middle layer and an equivalent amount of Al from the bottom layer moves into the middle layer leaving behind the impurities.
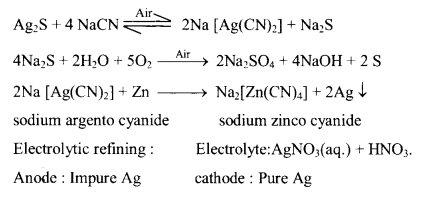
SILVER:
(a) Ores : Silver glance or argentite Ag2S, Ruby silver Ag2S. Sb2 S3, Horn silver AgCl.
(b) Process : Cyanidation or Mac-Arthur-Forrest process
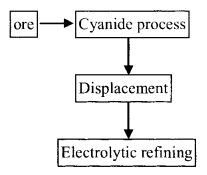
Reactions :
Seek help from the Onlinecalculator.guru and find all important formulas of chemistry subject along with formulae sheets, tables, and lists with examples.